Carbon-based Energy Carrier Research Team
We are engaged in the research and development of energy (hydrogen) storage technologies based on interconversion between CO2 and formic acid/methanol, for CO2 utilization.We are developing innovative systems to store and upgrade thermal energy at high temperature utilizing chemical reaction between hydrogen and metals.

Research themes
- Development of highly efficient catalysts that enable formic acid/methanol to be produced through carbon dioxide reduction (i.e., hydrogenation, electro-reduction), and that allow hydrogen to be produced from formic acid
- Investigation into technologies for producing high-pressure hydrogen from formic acid
- Development of thermochemical energy storage system and chemical heat pump utilizing reaction between hydrogen and metals

Members
SAITA Itoko
HIMEDA Yuichiro
CHANDRASEKARAN Srinivas
KUMAR Ravi
SINGH Uday Raj
IIJIMA Takashi
IIJIMA Norio
HIROSE Takuji
The aims in this team are development of highly efficient catalysts for interconversion between CO2 and formic acid/methanol (i.e., hydrogenation, electro-reduction, and dehydrogenation) and the high-pressure reaction process.

Production of Formic Acid and methanol by Reduction of CO2
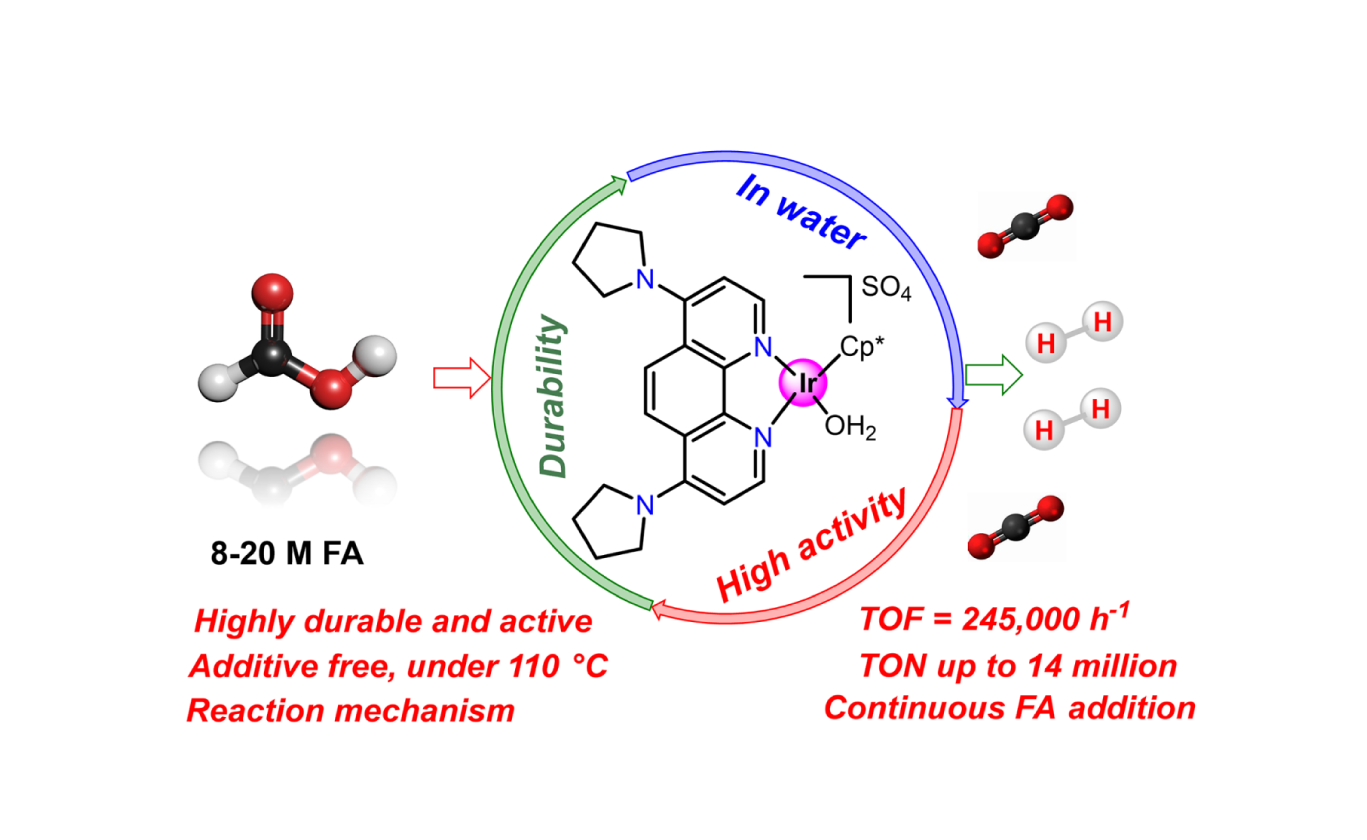
The catalyst that we have developed showed the highest performance for CO2 reduction (hydrogenation, electro-reduction) to formic acid and methanol. These catalysts can convert CO2 with high energy efficiency under mild reaction conditions.
High-Pressure H2 Production from Formic Acid
The high-performance catalysts which can supply high-pressure (> 1000 atm) and CO-free H2 by heating (<100 oC) of formic acid was developed. In addition, gas-liquid phase separation can easily separate of CO2 from the high-pressure system. The high-pressure H2 production from formic acid is original AIST’s technology.
Thermal energy storage utilizing reaction between metals and hydrogen
Thermal energy storage system was developed by utilizing reversible reaction between metals and hydrogen. The system can store thermal energy at temperature higher than 600℃ and supply thermal energy to industries with less CO2 emission.

Research
Highlights
Research Teams
- Thermal Energy Device Research Team
- Fundamentals of Ionic Devices Research Team
- Artificial Photosynthesis Research Team
- Carbon-based Energy Carrier Research Team
- Carbon Management Research Team
- Resource Circulation Technology Research Team
- Environmental Impact Research Team
- Environmental and Social Impact Assessment Team
- Data-Driven Smart Society Systems Research Team