Resource Circulation Technology Research Team
We seek to develop high-efficiency and environmentally friendly recovery technologies for critical metals that are indispensable for producing lithium-ion secondary batteries (LIBs), rare-earth magnets, catalysts, and other products.
Research themes
- Establishment of a simple rare-earth magnet recycling process
- Development of technologies for recovering rare-earth elements (REEs) from nontraditional resources and highly efficient technologies to recover platinum group metals
- Establishment of a new critical metal recovery process from waste LIBs
Research Team Leader / Greetings
NARITA Hirokazu
I have studied the separation of critical metals for many years, and I understand that this field is growing even more important owing to recent changes in international affairs and trends in decarbonization. We will contribute to the elimination of metal resource constraints in a zero emission society by establishing processes that can be put to practical use.
Members
OGATA Takeshi
KASUYA Ryo
SUZUKI Tomoya
KATASHO Yumi
OKI Tatsuya
HAYASHI Naohito
NAGANAWA Yuki
OMURA Naoki
FURUSHIMA Ryouichi
MURAKAMI Yuichiro
TANAKA Mikiya
KAYUKAWA Rie
SAITO Tomoko
YAGUCHI Miki
We seek to develop high-efficiency and environmentally friendly recovery technologies for critical metals (e.g., Li, Co, Ni, rare earth elements, and platinum group metals (PGM)) that are indispensable for producing lithium-ion secondary batteries (LIBs), rare-earth magnets, PGM catalysts, and other products.




Video
Research
Highlights
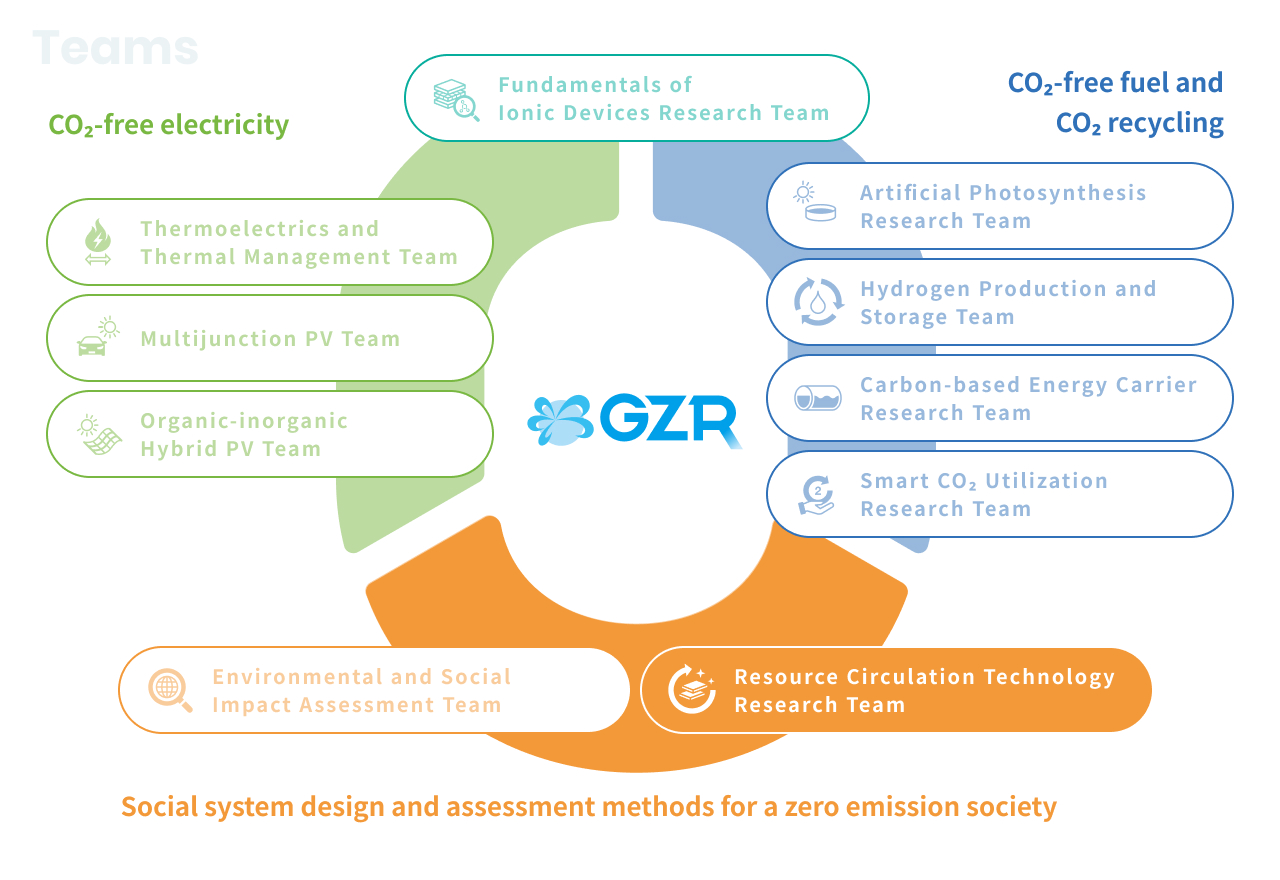
Research Teams
- Organic-inorganic Hybrid PV Team
- Multijunction PV Team
- Thermoelectrics and Thermal Management Team
- Fundamentals of Ionic Devices Research Team
- Artificial Photosynthesis Research Team
- Hydrogen Production and Storage Team
- Carbon-based Energy Carrier Research Team
- Smart CO2 Utilization Research Team
- Resource Circulation Technology Research Team
- Environmental and Social Impact Assessment Team